Graphite, molecular formula: C, molecular weight: 12.01, is a form of element carbon, each carbon atom is connected by three other carbon atoms (arranged in honeycomb hexagons) to form a covalent molecule. Because each carbon atom emits an electron, those that can move freely, so graphite is a conductor.
Graphite is one of the softest minerals, and its uses include making pencil leads and lubricants. Carbon is a non-metallic element located in the second cycle IVA group of the periodic table. Graphite is formed at high temperatures.
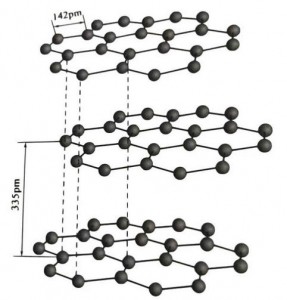
Graphite is a crystalline mineral of carbon elements, and its crystalline lattice is a hexagonal layered structure. The distance between each mesh layer is 3.35A, and the spacing of carbon atoms in the same mesh layer is 1.42A. It is a hexagonal crystal system with a complete layered cleavage. The cleavage surface is mainly molecular bonds, less attractive to molecules, so its natural float is very good.
In graphite crystals, the carbon atoms in the same layer form a covalent bond with sp2 hybridization, and each carbon atom is connected to three other atoms in three covalent bonds. The six carbon atoms form a six-continuous ring in the same plane, extending into a lamella structure, where the bond length of the C-C bond is 142pm, which is exactly within the bond length range of the atomic crystal, so for the same layer, it is an atomic crystal. Carbon atoms in the same plane have one p orbit, which overlap each other. Electrons are relatively free, equivalent to free electrons in metals, so graphite can conduct heat and electricity, which is the characteristic of metal crystals. Thus also classified as metallic crystals.
The middle layer of graphite crystal is separated by 335pm, and the distance is large. It is combined with van der Waals force, that is, the layer belongs to the molecular crystal. However, because the binding of carbon atoms in the same plane layer is very strong and extremely difficult to destroy, the dissolution point of graphite is also very high and its chemical properties are stable.
In view of its special bonding mode, can not be considered as a single crystal or polycrystal, graphite is now generally regarded as a mixed crystal.
Post time: Jul-31-2023